Dissertation
Click here if you are not redirected!.
Overview
View my dissertation in its entirety here.
Chapters/Projects
Introduction #1 – An accessible overview tuberculosis and lipids
Abstract:
This chapter explores the devastating illness of tuberculosis (TB) and the growing interest in host-directed therapy (HDT) as a potential avenue for novel anti-TB therapies. We aim to convey how one niche class of human lipids, sphingolipids, may affect the course of a Mycobacterium tuberculosis (Mtb) infection. We introduce TB, its historical context, and the evolution of mycobacteria alongside humans. We also introduce the crucial and enigmatic roles of sphingolipids throughout human biology and how they may present novel ways of treating TB infections. These historical contexts are essential for a deeper discussion on the interplay between Mtb infection and the lipids in the host cell. Further, it is necessary for individuals attempting to discover potential tuberculosis treatments to consider the disease’s historical background. A low-cost, effective therapy is a crucial first step in rectifying the long-standing systemic injustices of tuberculosis treatment.
Introduction #2 – TB & The Sphinx
Abstract:
Mycobacterium tuberculosis (Mtb) is a significant cause of infectious death worldwide. This intracellular pathogen uses many mechanisms to subvert the host immune system and lie undetected before causing active disease. This subtle pathogen is uniquely dependent on host lipids for energy, production of host-damaging materiel, and immune subversion. In the face of antibiotic-resistant Mtb strains, researchers have turned toward lipid biology for potential avenues of novel antitubercular therapy. The sphingolipid family has gained attention for its profound roles in determining cell fate, immune signaling, and antimicrobial activity. We review the state of the field regarding sphingolipids as candidate targets of host-directed antitubercular intervention.
Breaking and Entering: Sphingomyelin Biosynthesis Is Essential for Phagocytic Signaling during Mycobacterium tuberculosis Host Cell Entry
Abstract:
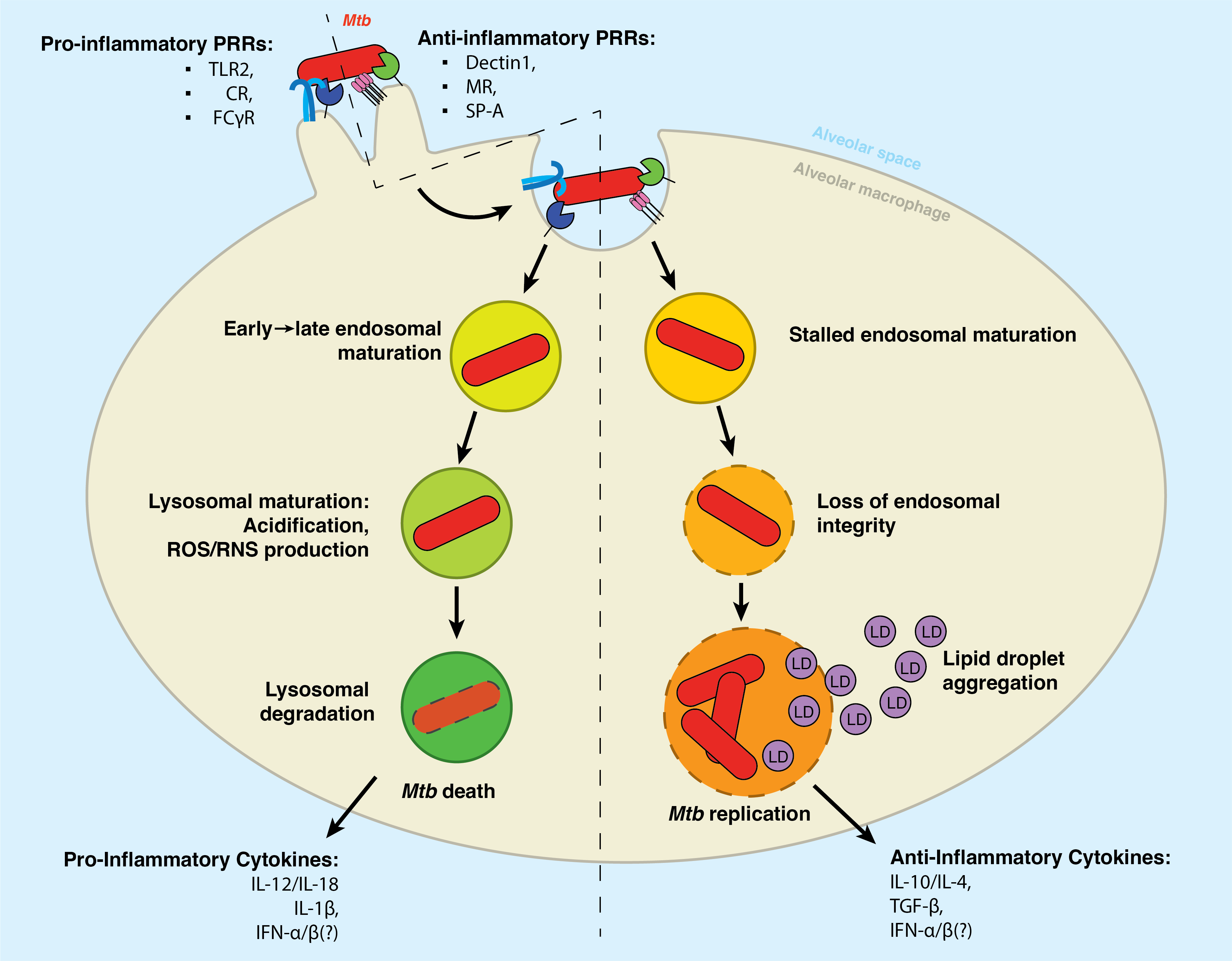
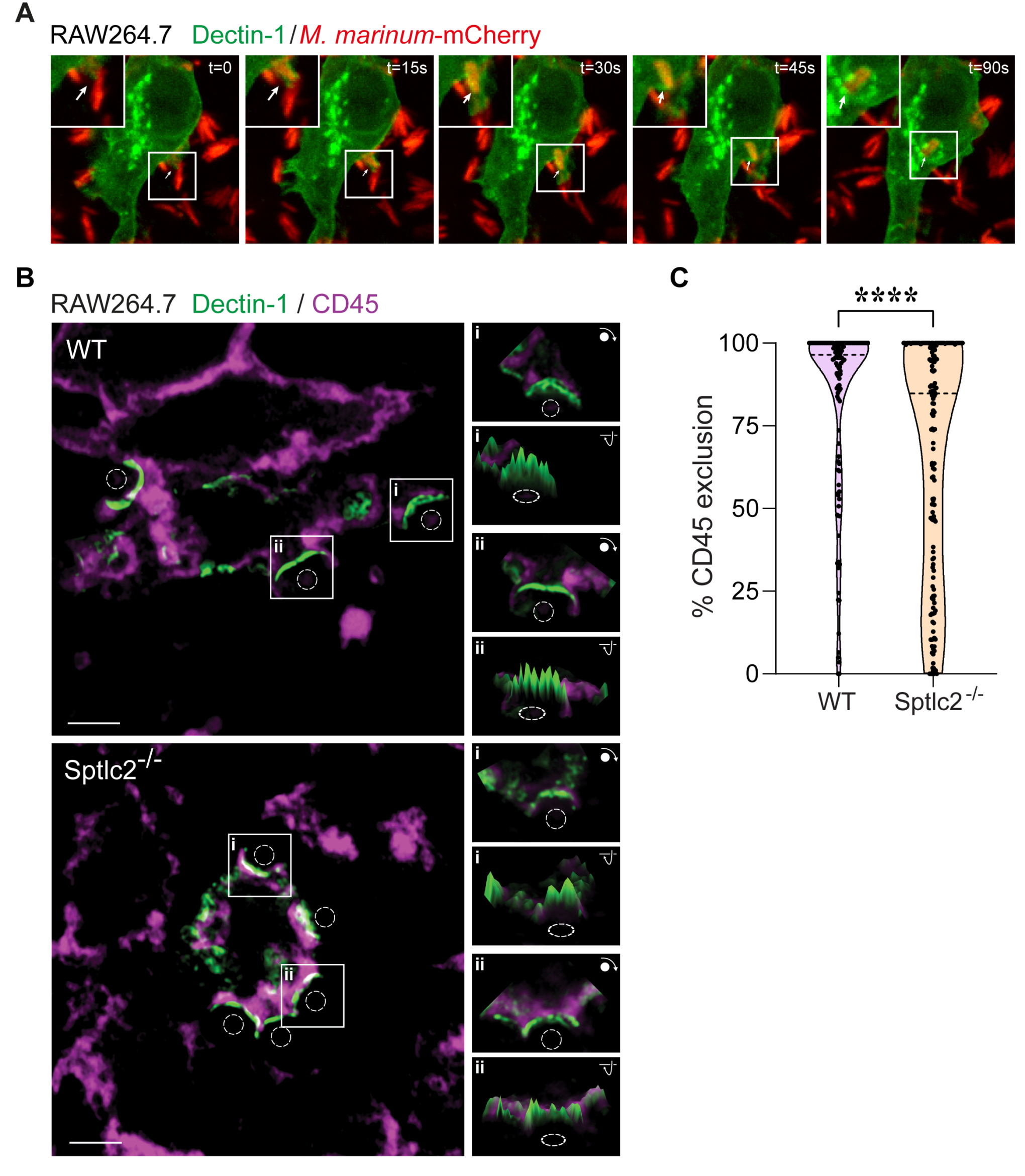
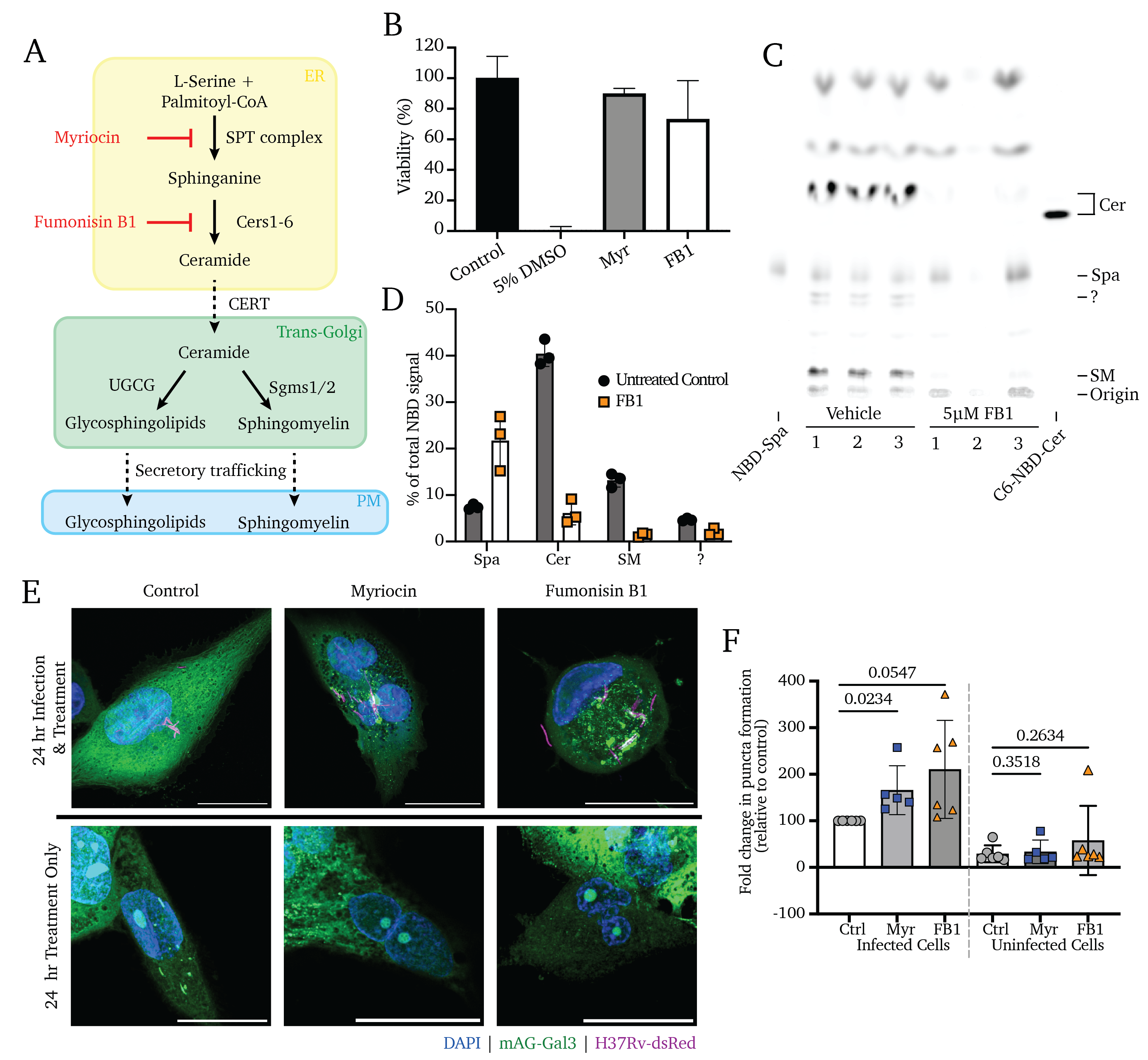
Phagocytosis by alveolar macrophages is the obligate first step in Mycobacterium tuberculosis (Mtb) infection, yet the mechanism underlying this process is incompletely understood. Here we show that Mtb invasion relies on an intact sphingolipid biosynthetic pathway. Inhibition or knockout of early sphingolipid biosynthetic enzymes greatly reduces Mtb uptake across multiple phagocytic cell types without affecting other forms of endocytosis. While the phagocytic receptor Dectin-1 undergoes normal clustering at the pathogen contact sites, sphingolipid biosynthetic mutant cells fail to segregate the regulatory phosphatase CD45 from the clustered receptors. Blocking sphingolipid production also impairs downstream activation of Rho GTPases, actin dynamics, and phosphoinositide turnover at the nascent phagocytic cup. Moreover, we find that production of sphingomyelin, not glycosphingolipids, is essential for Mtb uptake. Collectively, our data support a critical role of sphingomyelin biosynthesis in an early stage of Mtb infection and provide novel insights into the mechanism underlying phagocytic entry of this pathogen.
Sealing the Gaps: Sphingolipids are essential for repairing the endosomal damage caused by Mycobacterium tuberculosis
Abstract:
Tuberculosis infection remains one of the leading causes of death worldwide, causing over 1.3 million deaths in 2022 alone. With the emergence of antibiotic-resistant strains of Mycobacterium tuberculosis (Mtb), there is a pressing need for novel and improved therapeutics. Here, we characterize the critical role of sphingolipids in restricting intracellular replication of Mtb. Sphingolipids are essential for rapidly responding to membrane damage and maintaining lysosomal integrity during Mtb infection. Additionally, sphingolipids enable the restriction of Mtb growth within host cells. Understanding the influence of host sphingolipids on Mtb infection may reveal novel routes for host-directed therapy. This research provides valuable insights into the pathogenesis of Mtb infection and could pave the way for developing new therapeutic strategies.
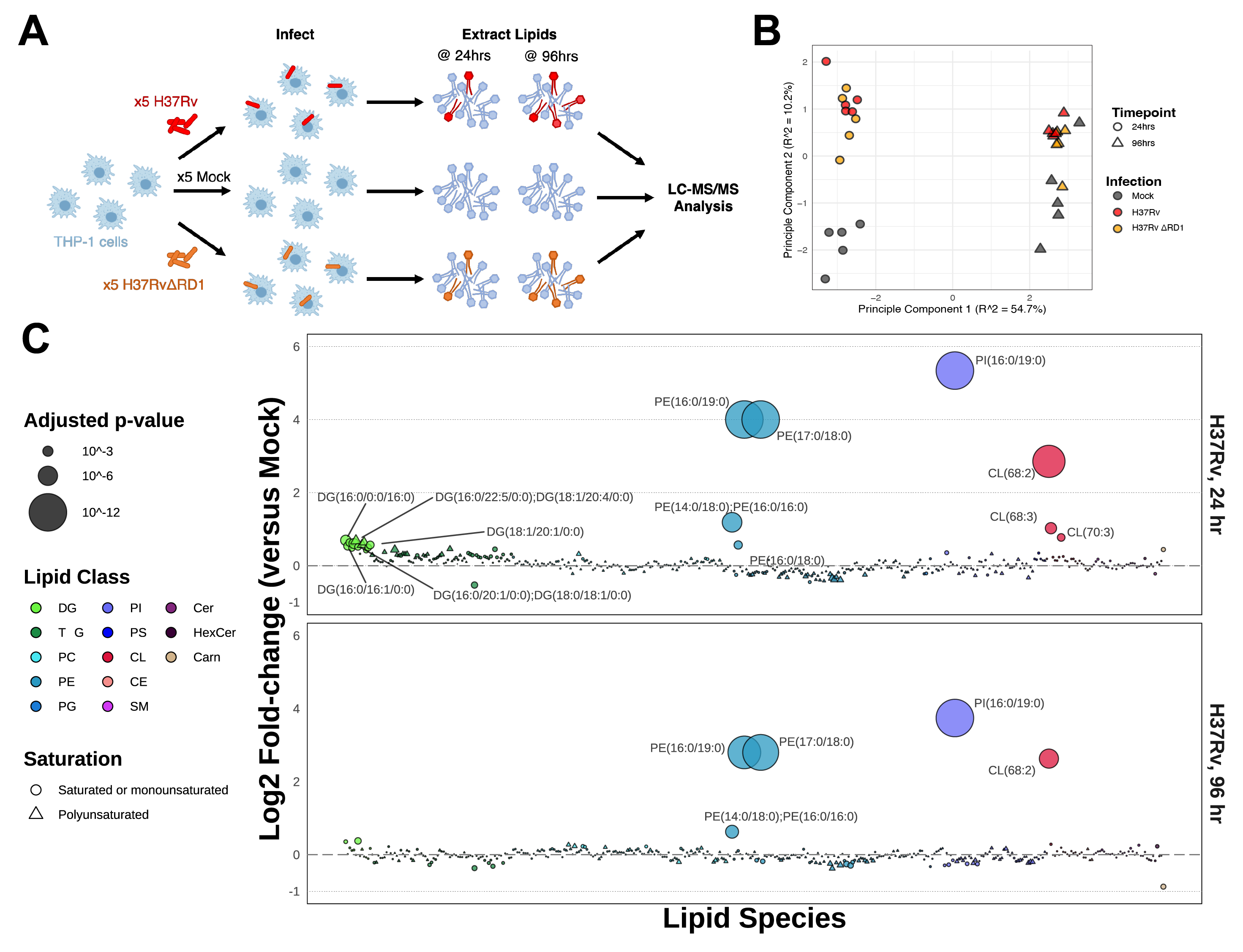
Living Off the Fat of the Cell: A lipidomics map of the Mycobacterium tuberculosis-infected cell
Abstract:
For centuries, humans have been seeking reliable treatment for tuberculosis, but unfortunately, it still poses a significant threat to global health. Recent research has shown that host lipid biology may offer new opportunities for developing novel antitubercular therapy. Here, we identify unique signatures of Mycobacterium tuberculosis (Mtb) infection by comparing the global lipidome of cells infected with pathogenic and attenuated Mtb strains against the lipidomes of cells treated with isolated Mtb cell wall components. We find that pathogenic and attenuated infections and treatments with pathogen-associated molecular patterns induce significant upregulation of diacylglycerol production. However, only pathogenic infection increases the production of triacylglycerol and acylcarnitine, reflecting induced fatty acid β-oxidation, while inhibiting the synthesis of lysophospholipids and plasmalogens, which reflects concerted subversions of the macrophage antimicrobial program. This research shows great potential in identifying potential host-directed therapies.
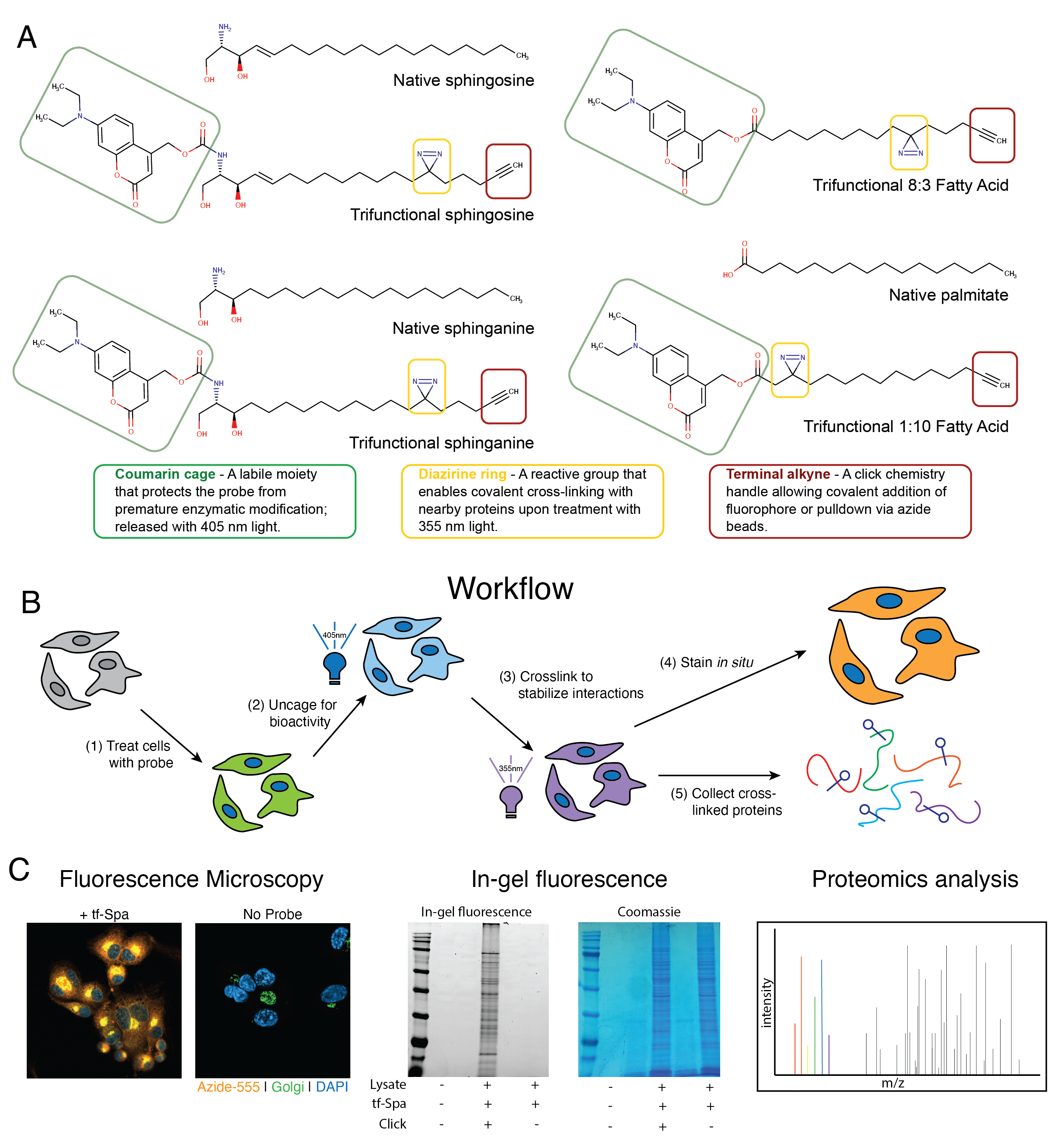
An Affinity for Sphingolipids: Trifunctional lipid probes reveal protein-sphingolipid interactions during Mycobacterium tuberculosis infection
Abstract:
Understanding the molecular interactions underlying Mtb’s capacity for immune subversion and hijacking of the host cell can provide a unique insight into potential new routes of antitubercular therapy. Because Mtb is a pathogen with a unique sensitivity to the host lipid compartment, and because sphingolipids are a lipid family with emerging roles in regulating antimicrobial functions in macrophages, we sought to characterize the sphingolipid interactome of an Mtb-infected cell. To this end, we utilized trifunctionalized lipid analogs as affinity handles for enrichment proteomics. We found that Mtb infection induces a significant reprogramming of proteins that interact with sphingosine. These changes included a downregulation of interactions among proteins involved in secretory trafficking and lysosomal maturation and an upregulation of interactions with cytoskeletal proteins. We further identify a candidate Mtb protein that selectively interacts with trifunctional sphingosine. This analysis is the first of its kind and represents a crucial step in characterizing how sphingolipids mediate the cellular response to intracellular infection.
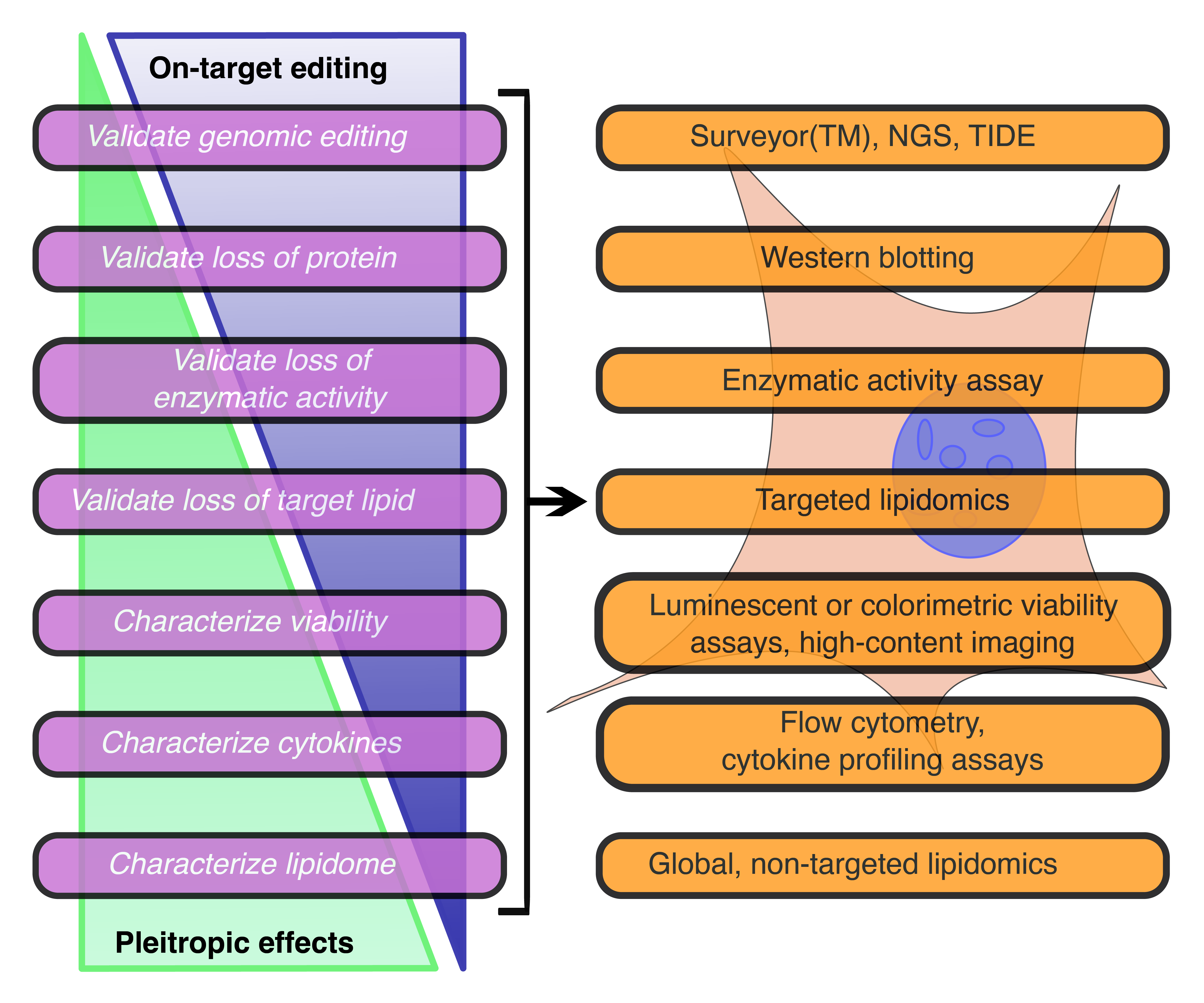
Genetic tools to study sphingolipids in disease
Abstract:
Sphingolipids are a critical family of membrane lipids with diverse functions in eukaryotic cells, and a growing body of literature supports that these lipids play essential roles during the lifecycles of viruses. While small molecule inhibitors of sphingolipid synthesis and metabolism are widely used, the advent of CRISPR-based genomic editing techniques allows for nuanced exploration into the manners in which sphingolipids influence various stages of viral infections. Here we describe some of these critical considerations needed in designing studies utilizing genomic editing techniques for manipulating the sphingolipid metabolic pathway, as well as the current body of literature regarding how viruses depend on the products of this pathway. Here, we highlight the ways in which sphingolipids affect viruses as these pathogens interact with and influence their host cell and describe some of the many open questions remaining in the field.
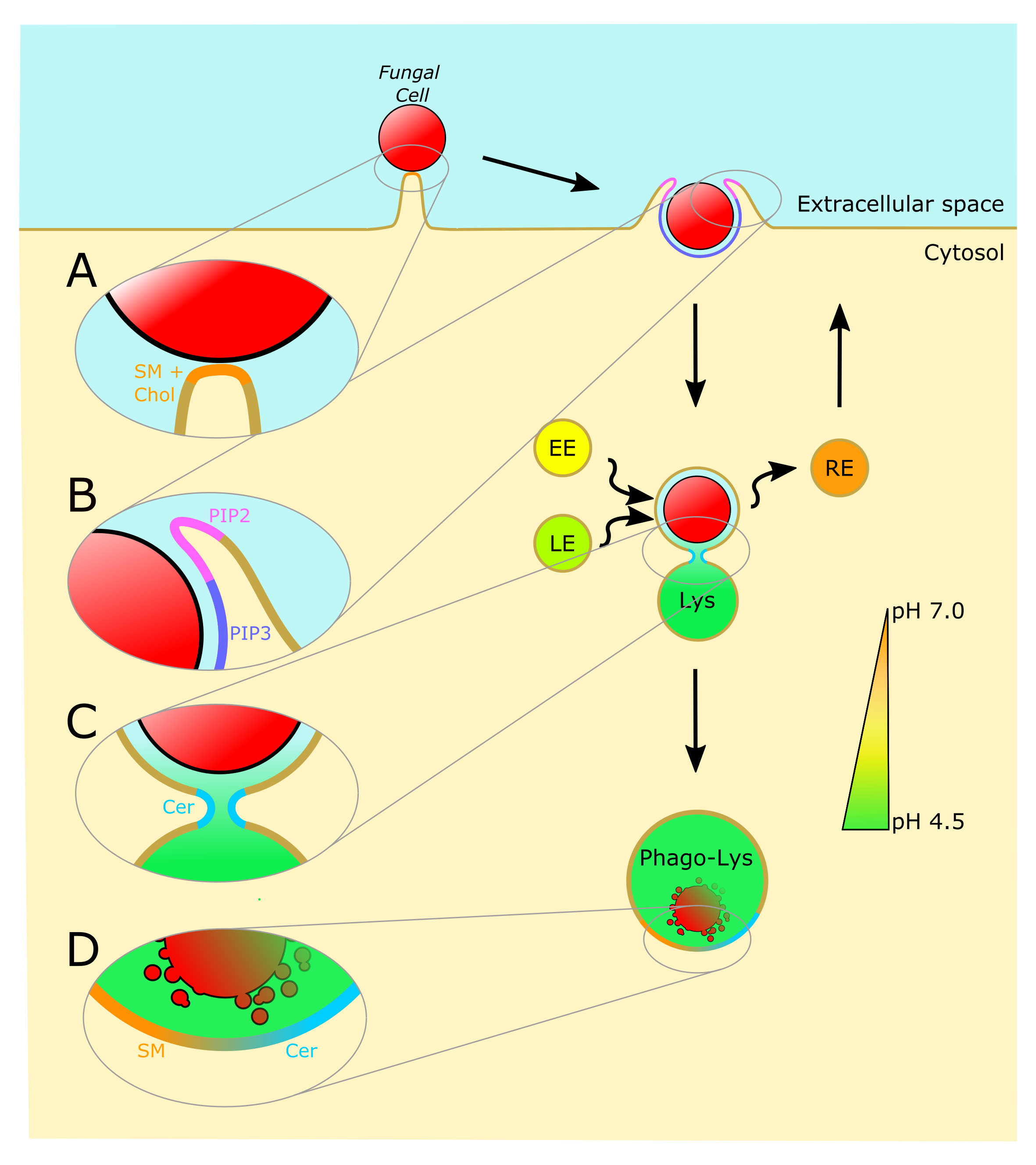
The Squeaky Yeast Gets Greased: The Roles of Host Lipids in the Clearance of Pathogenic Fungi
Abstract:
Fungal infections remain a global health threat with high morbidity and mortality. The human immune system must, therefore, perpetually defend against invasive fungal infections. Phagocytosis is critical for the clearance of fungal pathogens, as this cellular process allows select immune cells to internalize and destroy invading fungal cells. While much is known about the protein players that enable phagocytosis, the various roles that lipids play during this fundamental innate immune process are still being illuminated. In this review, we describe recent discoveries that shed new light on the mechanisms by which host lipids enable the phagocytic uptake and clearance of fungal pathogens.
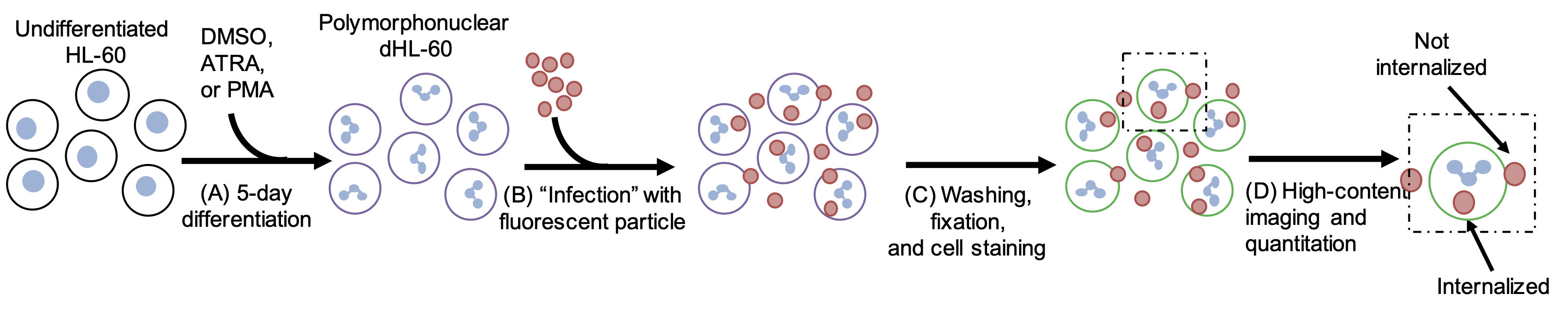
Rapid visualization and quantification of phagocytosis in neutrophils
Abstract:
Phagocytosis by phagocytes such as neutrophils is a crucial part of the host innate immune response against invading pathogens. Phagocytosis is a complex process that initiates with the binding of the particles on the cell surface of the phagocytes through the interaction of pattern recognition receptors with ligands on the surface of the pathogens. During this process, phagocytes undergo extensive membrane reorganization and cytoskeleton rearrangement at their cell surface. To gain better insight about the molecular mechanisms of this dynamic cellular process, visualization and quantification in a high-throughput manner is essential. Here, we describe a microscope-based method to visualize and quantify phagocytic uptake of pathogens (such as bacteria and fungi) and model particulates that are larger than 0.5 μm (such as Zymosan A and IgG-coated beads).
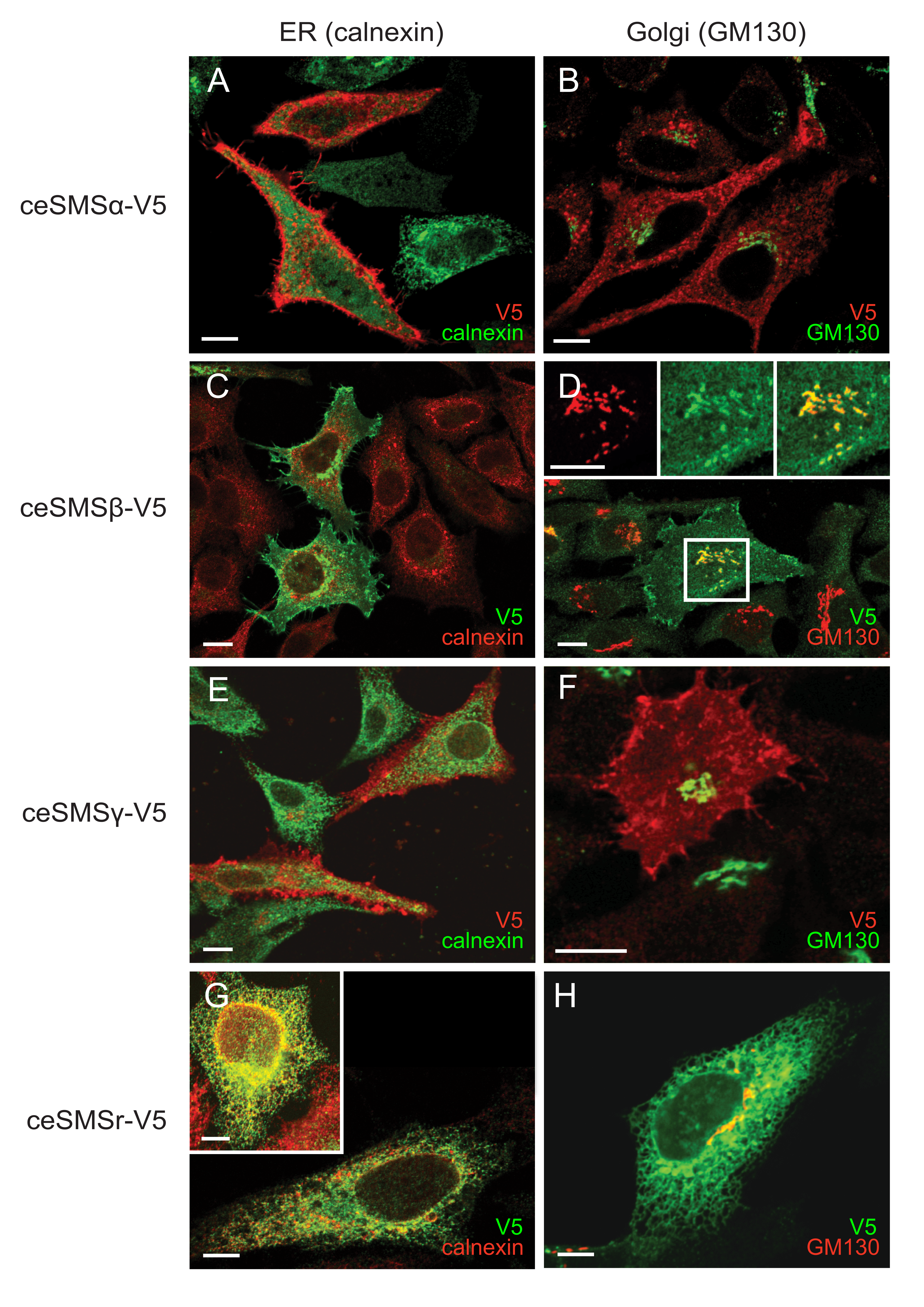
Systematic analysis of the sphingomyelin synthase family in C. elegans
Abstract:
Sphingomyelin (SM) is a major component of mammalian cell membranes and particularly abundant in the myelin sheath that surrounds nerve fibers. Its production is catalyzed by SM synthases SMS1 and SMS2, which interconvert phosphatidylcholine and ceramide to diacylglycerol and SM in the Golgi and at the plasma membrane, respectively. As the lipids participating in this reaction fulfill both structural and signaling functions, SMS enzymes have considerable potential to influence diverse important cellular processes. The nematode Caenorhabditis elegans is an attractive model for studying both animal development and human disease. The organism contains five SMS homologues but none of these have been characterized in any detail. Here, we carried out the first systematic analysis of SMS family members in C. elegans. Using heterologous expression systems, genetic ablation, metabolic labeling and lipidome analyses, we show that C. elegans harbors at least three distinct SM synthases and one ceramide phosphoethanolamine (CPE) synthase. Moreover, C. elegans SMS family members have partially overlapping but also unique sub-cellular distributions and together occupy all principal compartments of the secretory pathway. Our findings shed light on crucial aspects of sphingolipid metabolism in a valuable animal model and opens avenues for exploring the role of SM and its metabolic intermediates in organismal development.