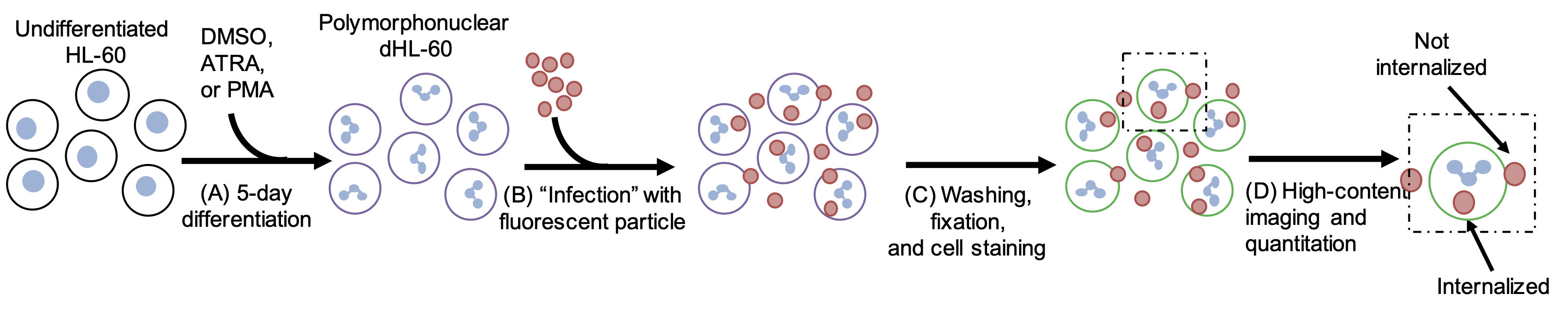
Dissertation Chapter 6: An Affinity for Sphingolipids
Trifunctional lipid probes reveal protein-sphingolipid interactions during Mycobacterium tuberculosis infection
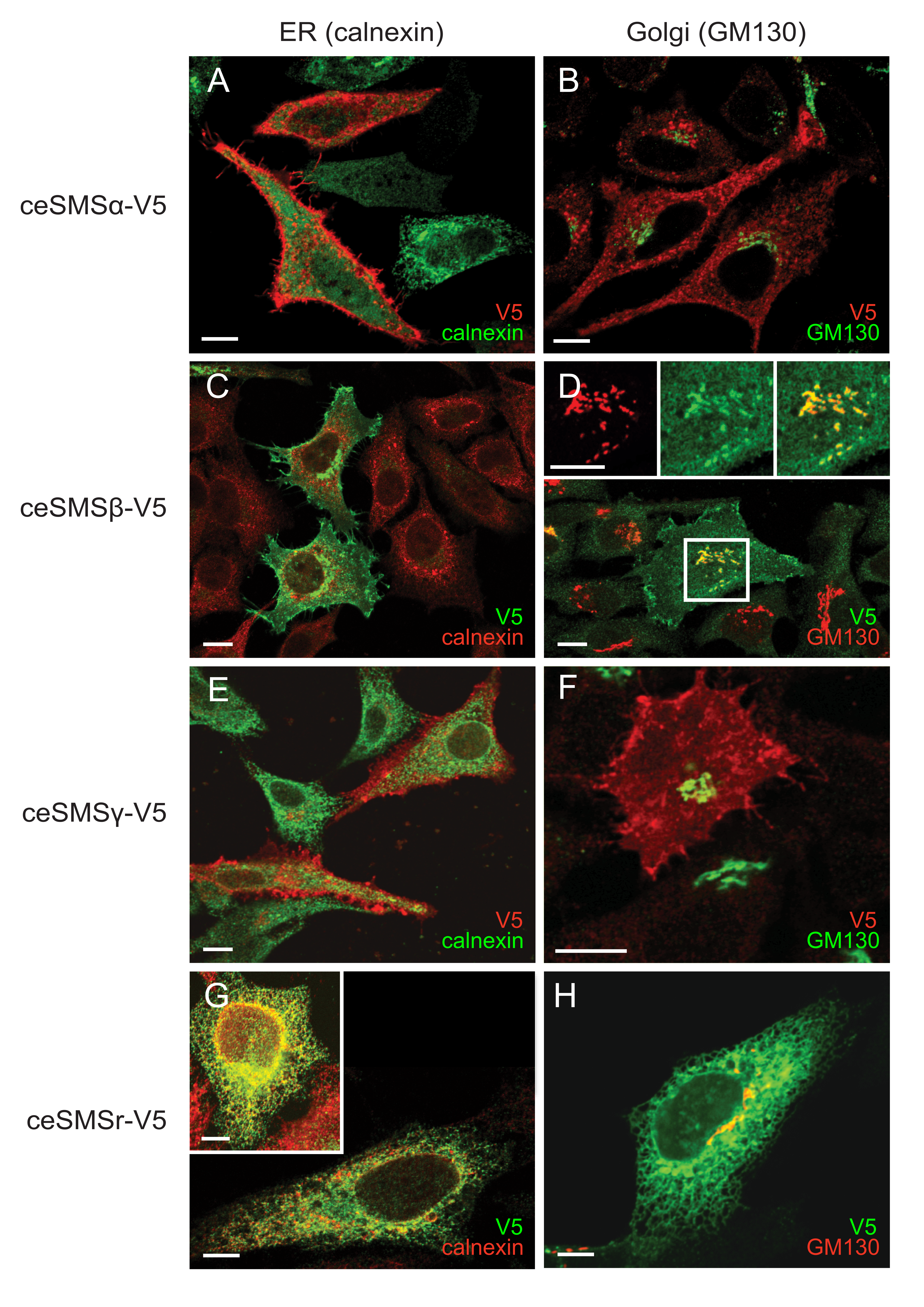
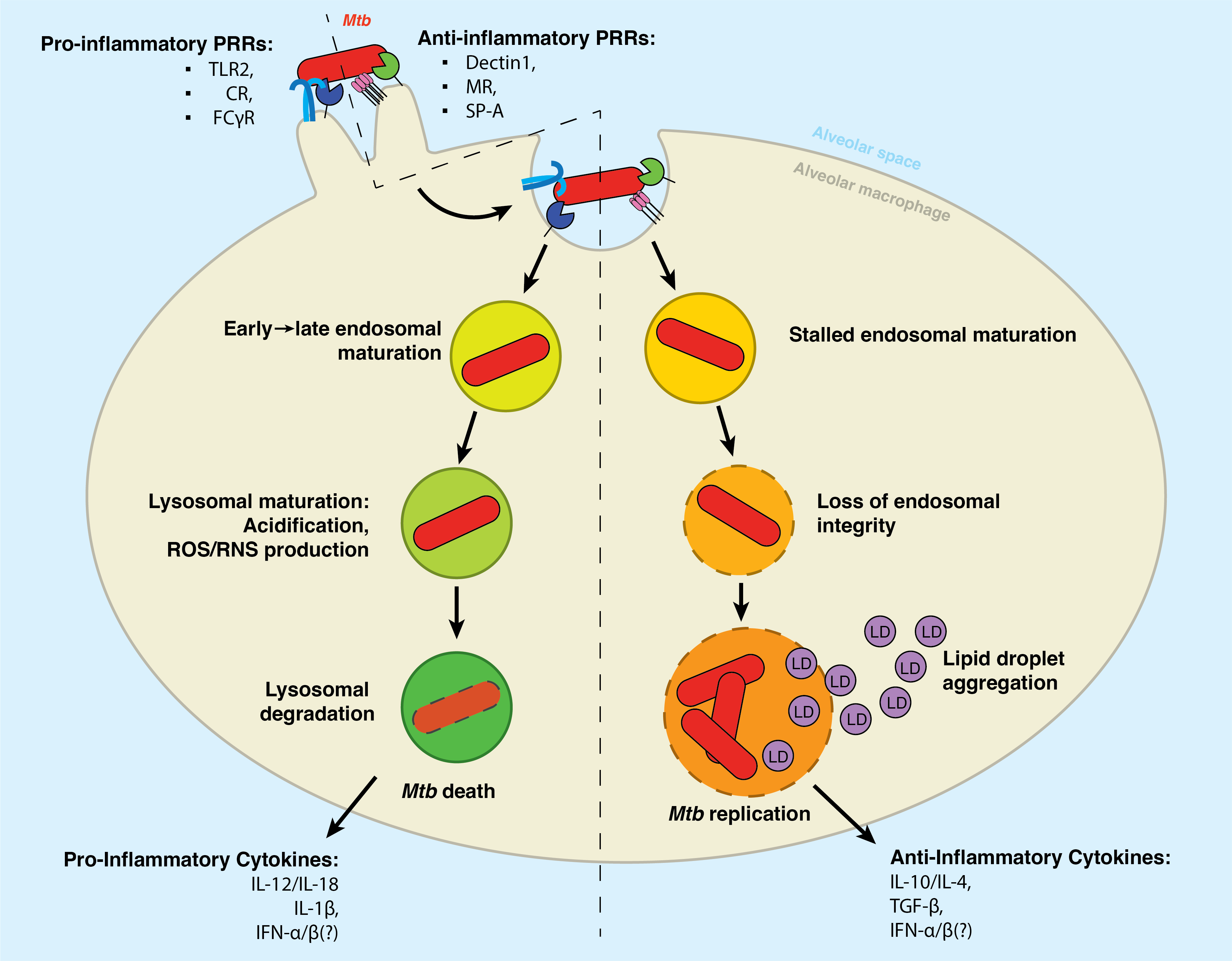
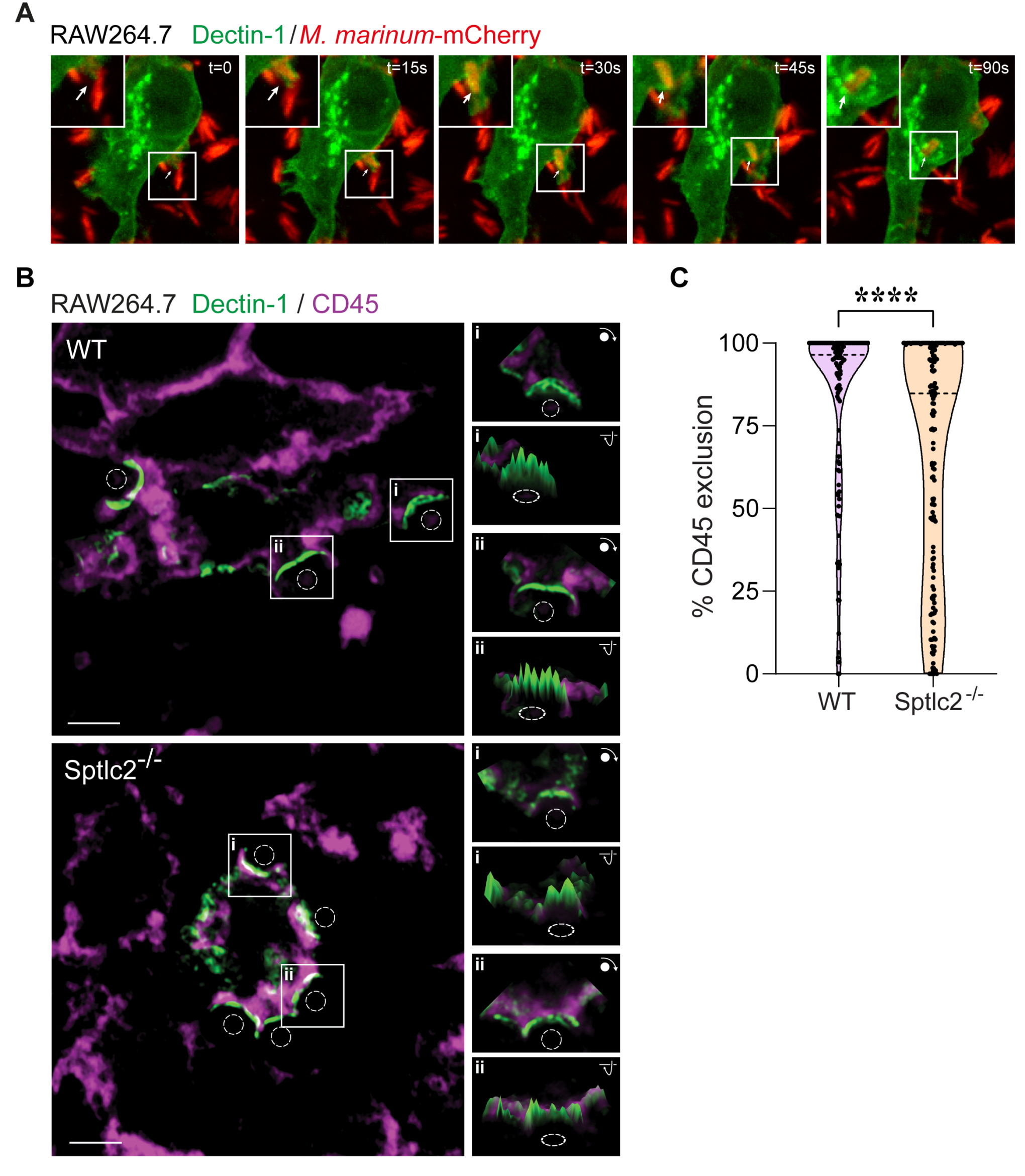
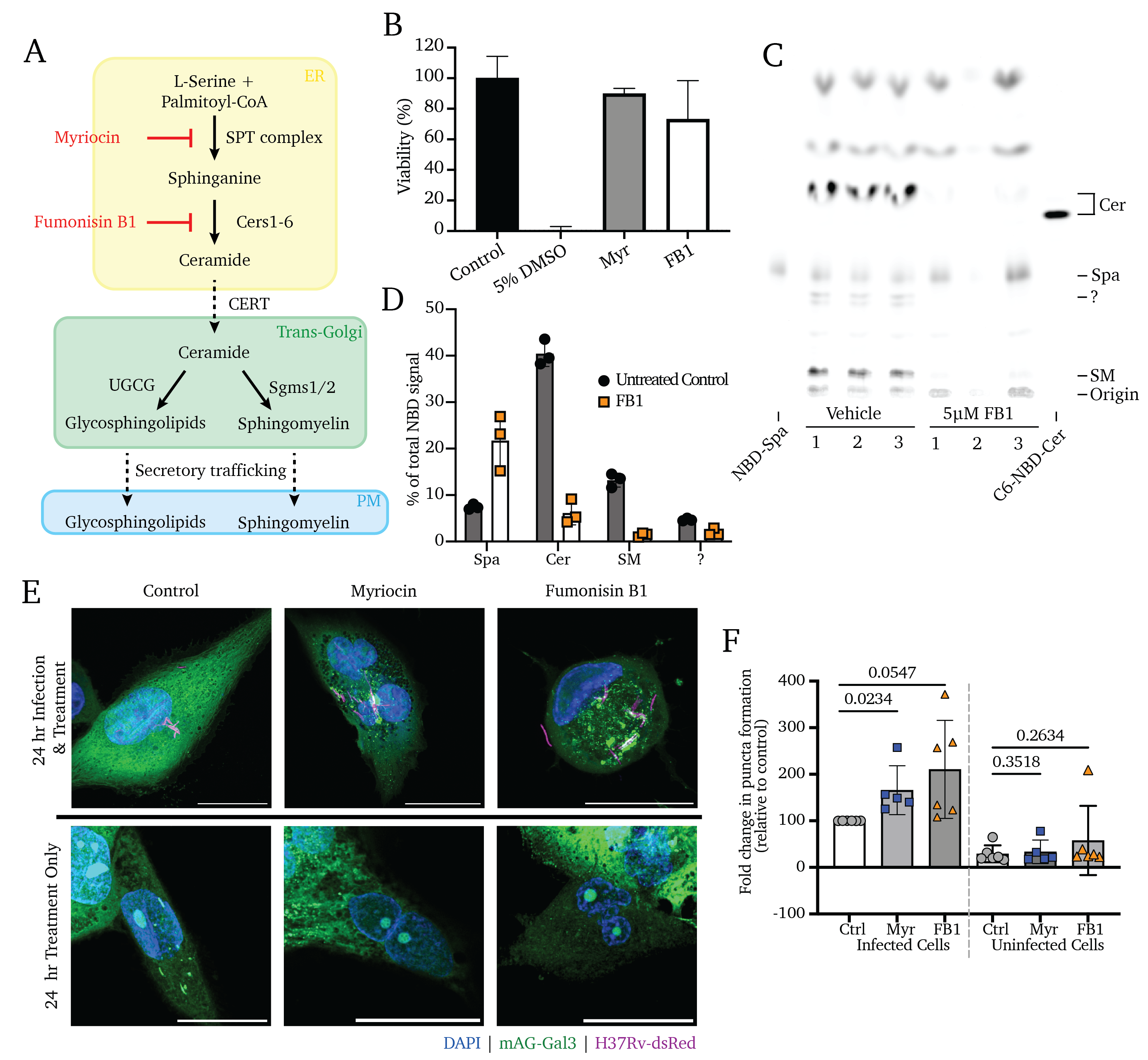
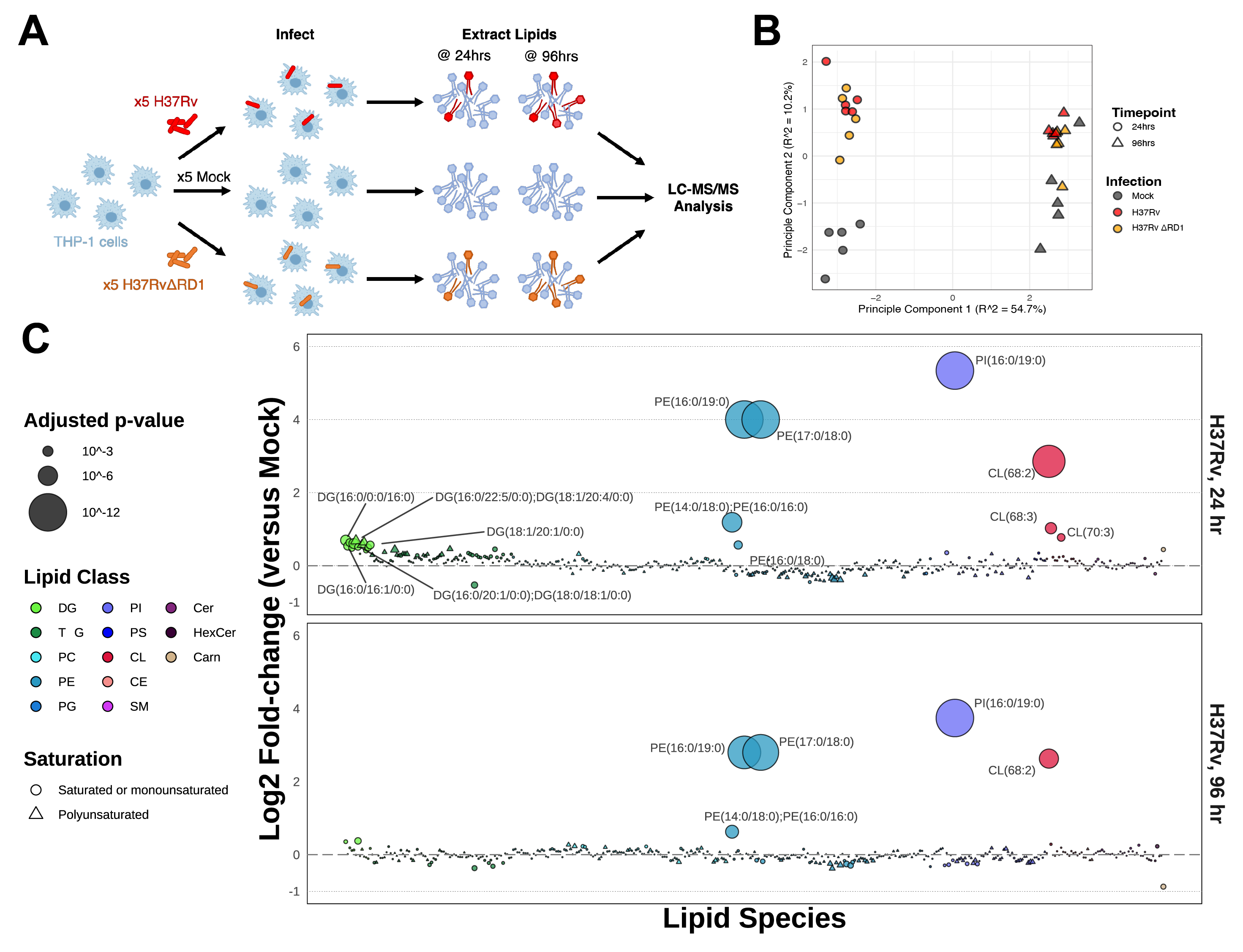
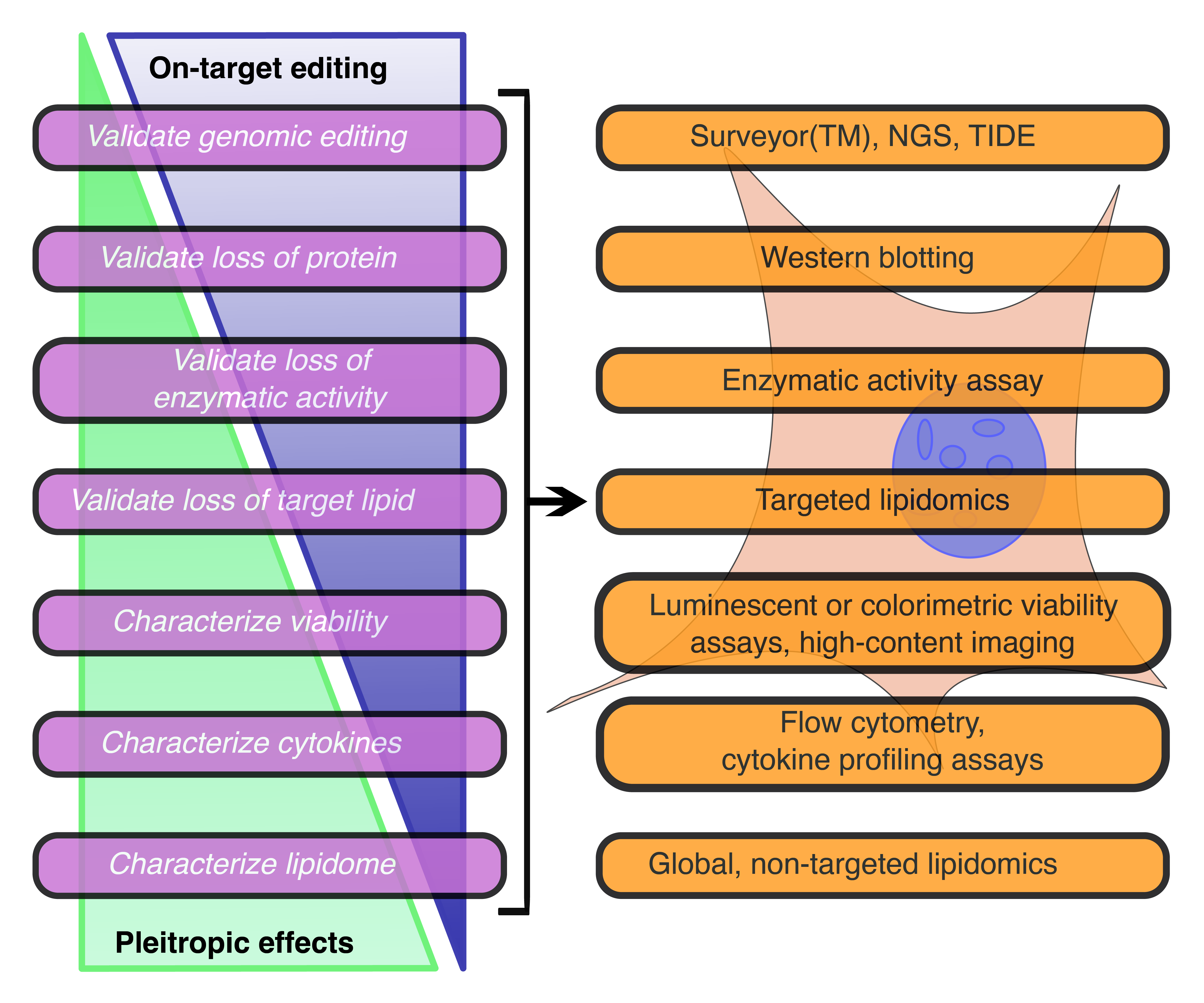
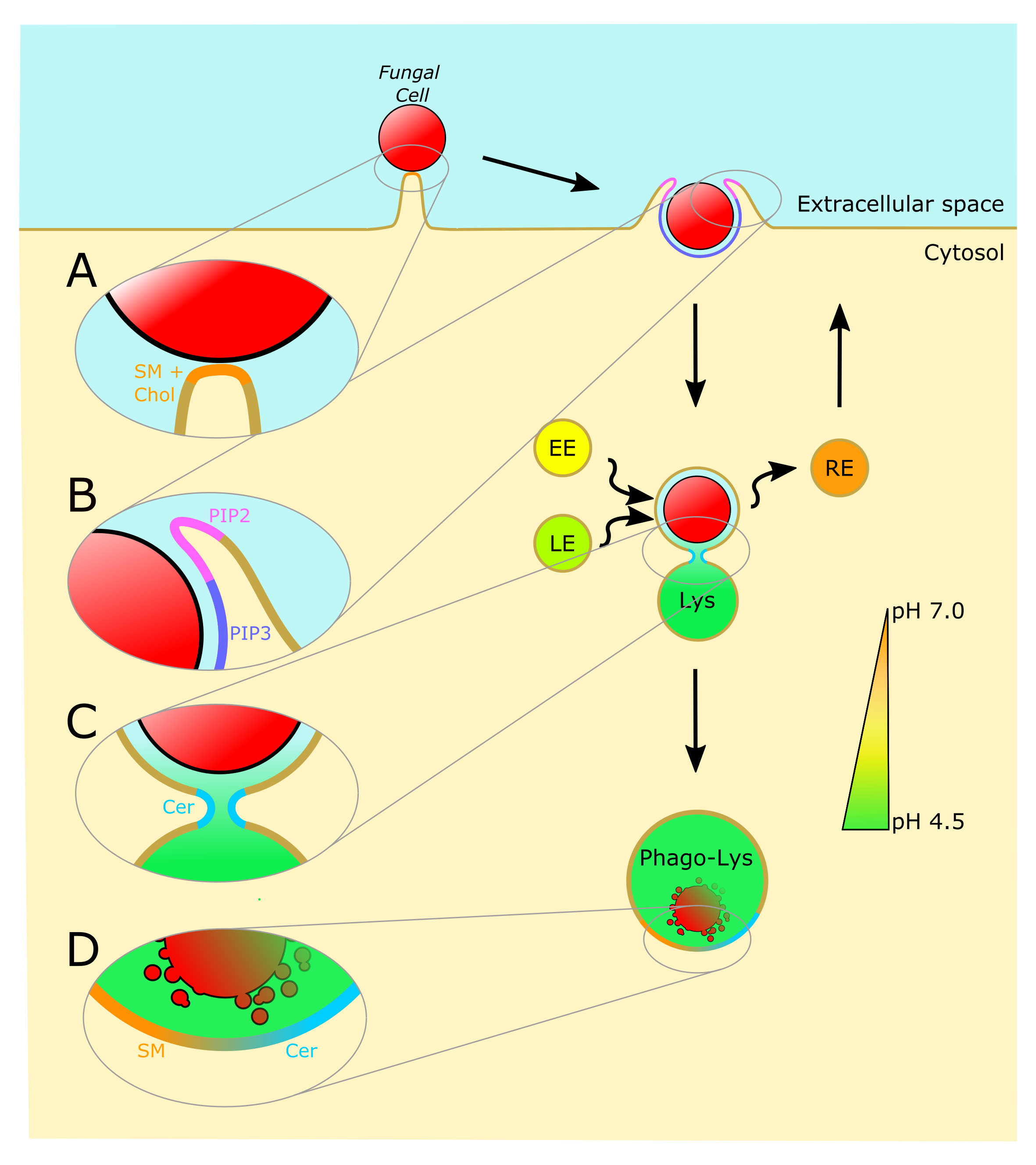
Understanding the molecular interactions underlying Mtb’s capacity for immune subversion and hijacking of the host cell can provide a unique insight into potential new routes of antitubercular therapy. Because Mtb is a pathogen with a unique sensitivity to the host lipid compartment, and because sphingolipids are a lipid family with emerging roles in regulating antimicrobial functions in macrophages, we sought to characterize the sphingolipid interactome of an Mtb-infected cell. To this end, we utilized trifunctionalized lipid analogs as affinity handles for enrichment proteomics. We found that Mtb infection induces a significant reprogramming of proteins that interact with sphingosine. These changes included a downregulation of interactions among proteins involved in secretory trafficking and lysosomal maturation and an upregulation of interactions with cytoskeletal proteins. We further identify a candidate Mtb protein that selectively interacts with trifunctional sphingosine. This analysis is the first of its kind and represents a crucial step in characterizing how sphingolipids mediate the cellular response to intracellular infection.
Abstract
Understanding the molecular interactions underlying Mtb’s capacity for immune subversion and hijacking of the host cell can provide a unique insight into potential new routes of antitubercular therapy. Because Mtb is a pathogen with a unique sensitivity to the host lipid compartment, and because sphingolipids are a lipid family with emerging roles in regulating antimicrobial functions in macrophages, we sought to characterize the sphingolipid interactome of an Mtb-infected cell. To this end, we utilized trifunctionalized lipid analogs as affinity handles for enrichment proteomics. We found that Mtb infection induces a significant reprogramming of proteins that interact with sphingosine. These changes included a downregulation of interactions among proteins involved in secretory trafficking and lysosomal maturation and an upregulation of interactions with cytoskeletal proteins. We further identify a candidate Mtb protein that selectively interacts with trifunctional sphingosine. This analysis is the first of its kind and represents a crucial step in characterizing how sphingolipids mediate the cellular response to intracellular infection.
Click here if you are not redirected!
No matching items